Naming Alkanes: An alkane is a hydrocarbon with only single covalent bonds. The general formula for an alkane is CnH2n+2, where n can be any whole number greater than 0. Alkanes are the simplest type of hydrocarbon, and they all have just one IUPAC name that is derived from their molecular formula. However, alkanes can be more complicated than just normal straight chain alkanes.
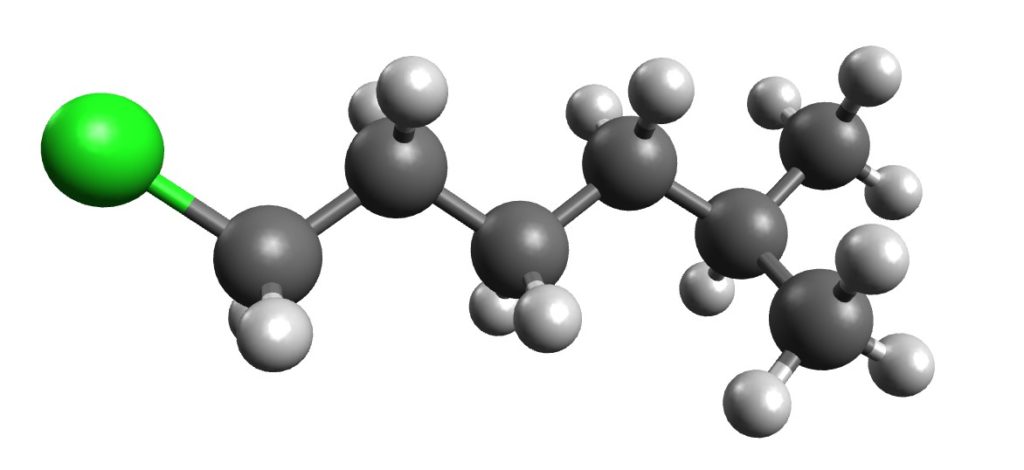
Alkanes use a simple naming system. Naming Alkanes: The Complete Guide
As with all of organic chemistry, nomenclature follows the IUPAC rules of naming. Please do not confuse this with their common names. For example, isopentane is a common name, the IUPAC name is 1-methylbutane, however both names refer to the same chemical compound.

Naming Alkanes: Nomenclature
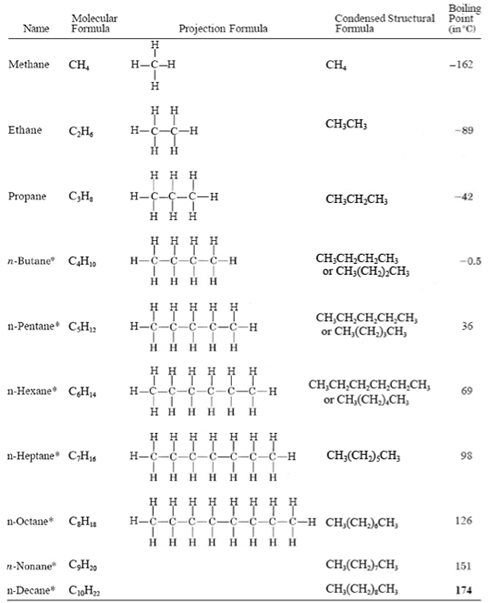
The very first thing that needs to be done is to start with the parent chain, which is the longest continuous chain of carbon atoms in the molecule. Once you have identified the parent chain, count the number of carbons in that chain. This will tell you what kind of alkane it is. You should also review the alkane formula before you get too far into this, the link is to one of our blog posts on it.
[PRO TIP: Circle the parent chain to easily identify it and separate it from the branches.] The parent chain can then be used to put together the alkane name in IUPAC format. It depends on your professor and your course, but most instructors would want you to know the names for all unbranched alkanes from 1 to 12 carbons:
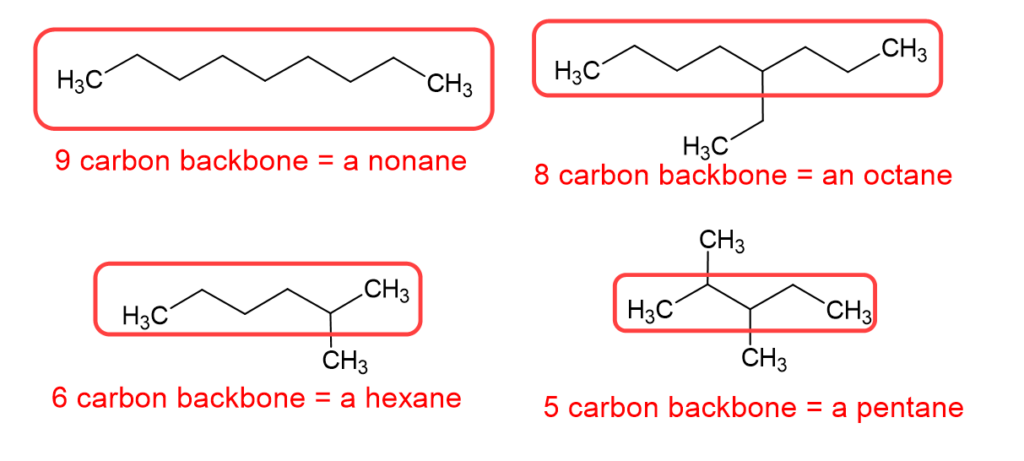
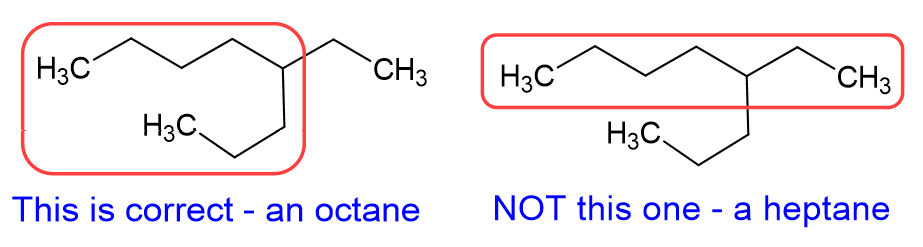
Let’s talk about that pro tip a little more. The best way to start a nomenclature problem, when it comes to alkanes, is to circle the longest continuous carbon chain, which is the carbon backbone, and then name the alkane accordingly. A quick note about the image below: please notice we call it “a” nonane or “an” octane. Octane itself, when it is eight carbon atoms in a straight chain, is called octane or n-octane. The octane we show below has a carbon branch, and would be name 4-ethyl octane, which is one of the isomers of octane, but is not n-octane.

How do you name alkanes with branches?
When you’re dealing with a molecule that is a little more complicated than a simple straight chain, we have a problem: We no longer can use the simple names based on number of carbons. The UIPAC name is one that names the backbone and then incorporates the substituents to give a name that is unique and distinctly identifiable to that molecule.
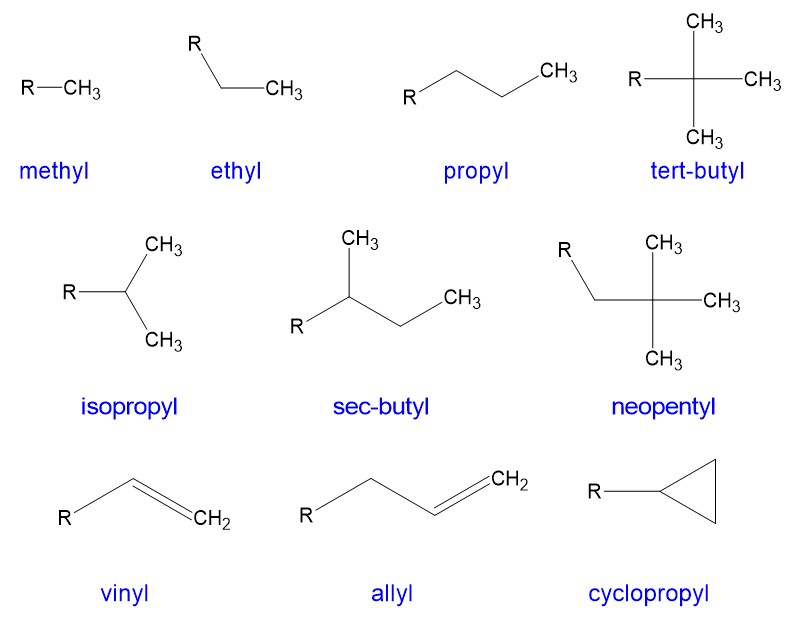
Below are the names of the most common alkyl branches (and some less common ones):
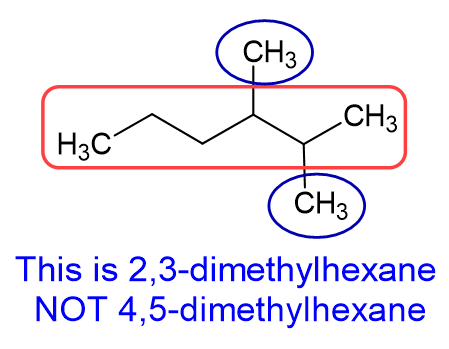
Now that we know the backbone alkane, and what the branches are, we need to determine the position of the branch. KEY PART: Number the branch so have the smallest number start the naming. As shown in the example below, this molecule is 4-ethyl octane NOT 5-ethyl octane. We need to count from the right side in this case, not the left side, which gives the lower number to the ethyl branch.
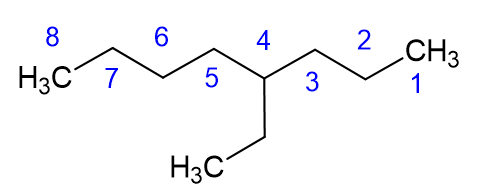
Here are a few other examples:

How do you name alkanes with substituents?
For this discussion, we are going to make a distinction between carbon branches and substituents. Technically, anything coming off of the carbon chain can be a substituent, but in an effort to be a little more clear, we are going to call things other than carbon branches “substituents”.
The most common one of these that you will find are halides. When halides are substituents on a substituent on a carbon chain, the molecule is called an alkyl halide. The halogen substituent gets the “o” suffix, as shown below, and gets a higher priority than a carbon branch. [we will talk more about priority in the next section]
| Halogen | Suffix |
| -F | “Floro” |
| -Cl | “Chloro” |
| -Br | “Bromo” |
| -I | “Iodo” |
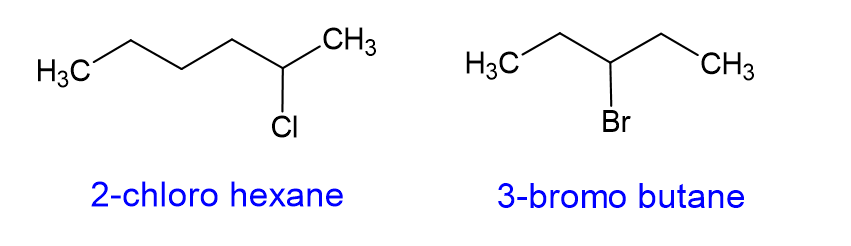
How do you name cycloalkanes?
Cycloalkanes are just alkanes in a ring. It is as simple as that. They are named according to how many carbon atoms they have in their ring. For example, cyclopropane is made up of three carbon atoms. The prefix cyclo- refers to the fact that the alkane is in a ring formation. The cycloalkanes (up to 10 carbons) are depicted below:

Quick note on these rings: As you well know, these rings are not actually flat, like they are shown on this page. They each have a preferred conformation, as you will see in great detail with cyclohexane as you proceed in organic chemistry. In fact, here is a conformation of cyclooctane, viewed in the Newman representation. Notice how it has axial and equatorial substituents, just like cyclohexane.
How do you name cycloalkanes with substituents and/or branches?
Simple, we name these the same way that we name straight chain alkanes. Here are the steps:
- Determine the longest number of carbon atoms. NOTE: If the longest chain of carbons is a straight chain alkane, then that’s how we name it and the cycloalkane is a substituent. Conversely, if the longest chain of carbons is a cycloalkane then the straight chain alkane is the substituent.
- Circle the cycloalkane in red, and number so that the substituents have the lowest total number possible.
- Place the substituents in alphabetical order, even if there number doesn’t come first in the order.
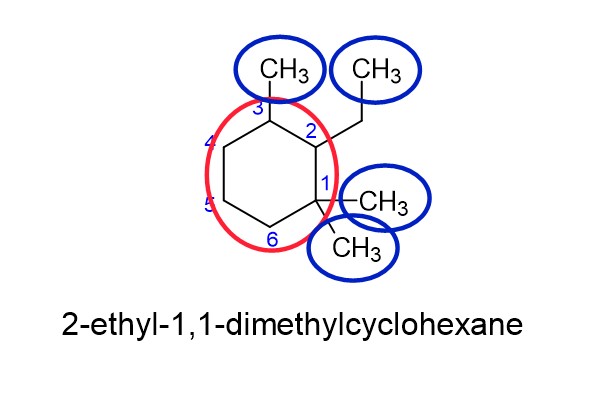
PRO TIP: When naming organic molecules, commas only go between two (or more) numbers, everything else gets dashes.
These are all alkanes and cycloalkanes, unless…..
There is a hydroxyl group present. That takes priority over alkyl side chains and halogens and turns the molecule into an alcohol. For example:
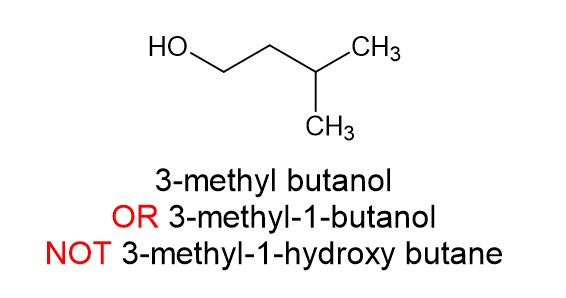
Common mistakes to avoid:
Common mistake #1: The longest alkane chain wasn’t the straightest on the page. This is a HUGE trick professors always try to pull on students. Here is an example of that:
Common mistake #2: Misnumbering the substituents. It is easy to forget that you DO NOT just got right to left (or left to right) to number substituents. It all depends on what gives you the lowest combination of numbers.
Common mistake #3: Cyclomethane and cycloethane don’t exist. The smallest cycloalkane is cyclopropane, and even that is pretty unstable because of strain. Fun fact: Cyclopropane is a triangle shape.

Common mistake #4: When numbering cycloalkane substituents, be sure to go around the ring and get the lowest number for each substituent. For example, the molecule below is 2-bromo-1-chloro-3-methylcyclohexane. If we numbered the other way around the ring, it would not have the lowest set of numbers possible. Also, we numbered the chlorine first to give it the lowest set of numbers. If we numbered the bromine first, it would give a higher set of numbers around the ring:

Common mistake #5: When naming the compound, be sure to put the substituents in alphabetical order, even it means putting a higher substituent number in front of a lower one.

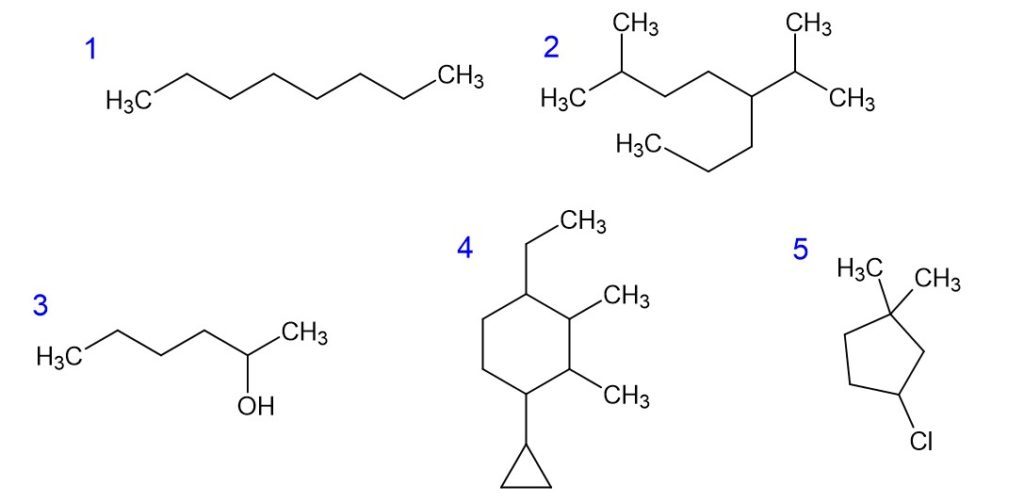
Naming Alkanes: Exercises—Scroll below for the answers
Last, one of favorite blog posts is about the top organic chemistry websites, is is worth a read.

- n-octane
- 2-methyl-5-isopropyloctane
- 2-hexanol
- 4-ethyl-2,3-dimethyl-1-cyclopropyl-cyclohexane
- 3-chloro-1,1-dimethylcyclopentane

