Epoxidation of Alkenes
Somewhere in one of your exams, you will see at least one question on epoxidation of alkenes. Quick tip: Before you start on alkenes, make sure you are good with alkanes, specifically the naming alkanes.
The reaction: What is epoxidation? An epoxide is a 3-membered ring containing two carbon atoms and one oxygen atom. Many students like to remember it as a cyclic ether. It is interesting because it is easily opened due to small ring strain and due to the electronegativity of the oxygen atom.

The reagents and starting materials:
1) What is an alkene? An alkene is an unsaturated hydrocarbon containing at least one double bond. They are ubiquitous (i love snooty words) in organic chemistry. The double bond itself has two parts, a sigma bond which holds the atoms together and a pi bond which strengthens in overall bond through added electron density, making them electron rich.

2) What is epoxidation of an alkene? The epoxidation reaction is where an alkene is subjected to a peroxyacid to convert it into an epoxide. Another way to say it is epoxidation is the electrophilic addition of oxygen to the double bond of the alkene.
3) What reagents can you use to create the epoxide? Generally, peroxy acids are used in this electrophilic addition to the alkene. A peroxy acid is like a carboxylic acid, but has two oxygen atoms bonded to each other.
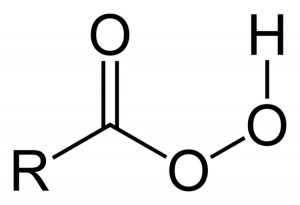
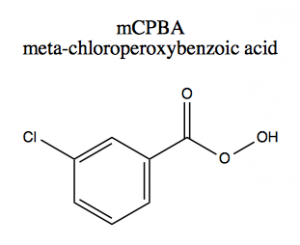
There are several types of commonly used peroxyacid such as proxy trifluoroacetic acid, peroxyacetic acid, hydrogen peroxide, and mCPBA, which is the most common of all. It is a peroxyacid and is shown below:
HOWEVER, there is another reagent, OsO4 , which also can give epoxides, but will give the opposite stereochemistry. More on that below.

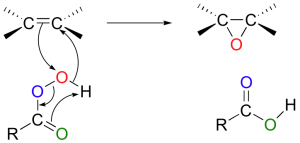
The mechanism: The mechanism for the reaction is relatively complex and may (or may not) be something your professors want you to know about, but hell, it’s good practice. While it is considered a single step reaction, it involves several changes. The double bond is our nucleophile and attacks the more electrophilic oxygen. This breaks the weak oxygen-oxygen bond and creates a new carbonyl. Once this carbonyl is formed, rearrangement occurs and the more electrophilic oxygen is released to become the oxygen of the epoxide.
The stereochemistry: The stereochemistry associated with this reaction is interesting and important. As the reaction can occur on a cis or trans alkene, we see the two different products come from these two different starting materials. The oxygen can only attack from one face of the alkene. This means that the stereochemistry of the alkene is retained. Translation: if you start with a cis alkene you will get a cis epoxide. If you start with a trans alkene, you will get a trans epoxide.
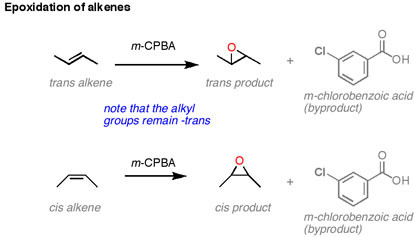
Further, remember that if you start with a di or tri-substituted alkene, you very well may create new stereocenters. However, if you remember the golden rule of chirality, you will know that you need to start with chirality in order to finish with it. These alkenes are not chiral to start with, therefore we will end with a racemic mixture. If there is some chirality in the molecule, somewhere near the double bond then that chirality can influence which face the peroxyacid is attacked from, but will not exclusively give a chirally pure product.

The reaction the reaction is versatile, and works on many different alkenes. Please remember that the reaction will not work on the double bonds of an aromatic compound.
The alternative reaction uses OsO4 . Since Osmium tetroxide is toxic and costs a ton of money it is not the preferred reactant for these kinds of epoxidation reactions. Catalytic osmium tetroxide and stoichiometric amounts of a decent oxidizing agent (such as H2O2) are used to make it less dangerous. Although syn diols will result from the reaction of KMnO4 and an alkene, potassium permanganate is less useful since it gives poor yields of the product because of overoxidation. For this reason, we have focused on the peroxy acids in this article, however, please remember that on your exam, OsO4 is a good reagent to obtain syn diols.
Some peroxy acid examples:
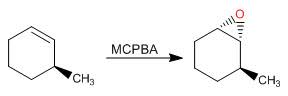
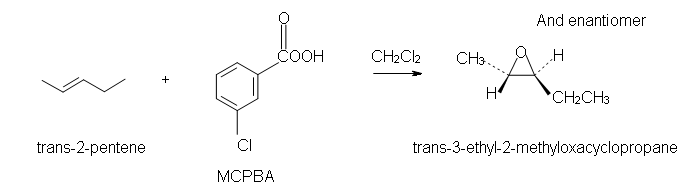
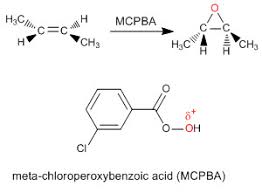
What can you do with epoxides once you have it? Great question! Epoxides, because they are so strained, are easily opened and can form other products. The first one that comes to mind is that epoxides can be opened up into trans-diols. It is a simple reaction, but highly useful, and usually the way you need to make a trans-diol on one of your exams.
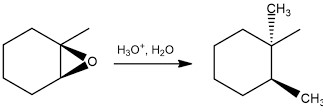
We rate the importance of this reaction, the epoxidation of alkenes, as four beakers out of five. This means you should know epoxidation reaction and the products you can get from it, but maybe don’t need to know the ins and outs of the mechanism for it. BE SURE to ask your prof though!

Reference: NIH epoxidation in the human body

