The Alkane Formula
Before we can answer the question of what is the alkane formula, we have to ask ourselves what is an alkane. An alkane is a simple hydrocarbon containing carbon and hydrogen single bonded to each other, with a carbon backbone. Any molecule with this structure is going to have the formula CnHn+2, where n is any integer. Let’s look at some examples of this:
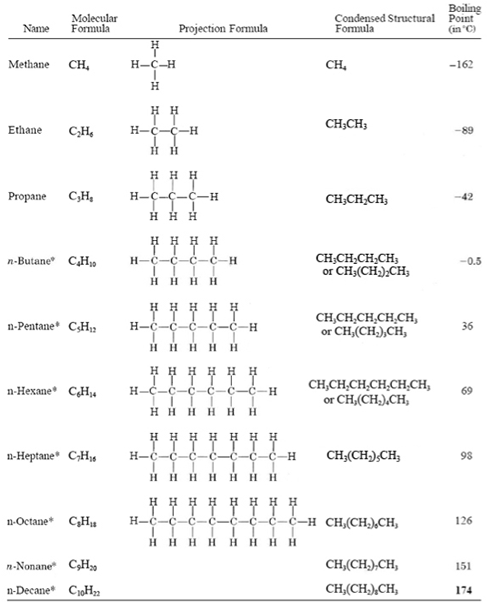

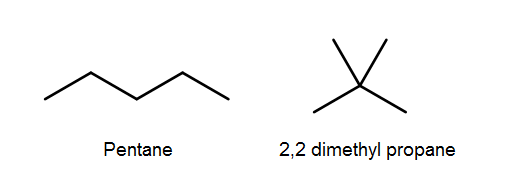
Please note, that this formula doesn’t tell us anything about the structure of the alkane, it only tells us that is fully unsaturated. For example, both pentane and 2,2-dimethylpropane will both be fully unsaturated and have the same molecular formula, but completely different structures and physical properties therefore we have to be very careful not to make assumptions about the structure of an alkane just based on its formula.
It’s a nice trick to know that any time you see a molecule with the formula CnHn+2, you know it is a fully saturated hydrocarbon, meaning that there are no double or triple bonds only single bonds. The degree of unsaturation, or “some of double bonds and rings” (SODAR) can be expressed using a similar formula. Here is a great post discussing the SODAR formula.

A quick rule of thumb to be able to determine if a molecule is saturated or not is just to ask if there are more than double the number of hydrogens as compared to carbon in the molecule. For example, if you have a hydrocarbon with the formula C5H12, we know that there are more than double the number of hydrogen therefore this is saturated. If we have a structure with the formula C5H10, there are only double the number of hydrogens therefore this is unsaturated at some point in the molecule. What this little rule of thumb doesn’t tell us is where that unsaturation is or what type of unsaturation it is, a double bond or a ring.

