What is the Lewis Structure of C2H4O?
What are the Lewis Structures of C2H4O? Actually, there are several plausible structures. This molecular formula lead to either acetaldehyde, Ethylene Oxide, or Vinyl Alcohol. All are correct answers. Below, we show two methods to arrive at any one of these answers.
What are these molecules?
Acetaldehyde is colorless liquid with a pungent, fruity odor and is an important intermediate in various chemical reactions and is used in the production of many chemicals and materials. Acetaldehyde is primarily known for its role in alcohol metabolism. When alcohol is consumed, it is first broken down by the liver into acetaldehyde before being further metabolized into acetic acid and eventually carbon dioxide and water. The buildup of acetaldehyde in the body is what leads to some of the more unpleasant effects of alcohol consumption, such as hangovers.
Ethylene oxide is a flammable and colorless gas at room temperature, but it is commonly stored and transported as a liquid due to its low boiling point. Ethylene oxide has a sweet, ether-like odor. Ethylene oxide is an extremely versatile chemical and is primarily used in the production of other chemicals. It is a key building block for the synthesis of various products, including plastics, resins, solvents, detergents, textiles, and pharmaceuticals. It is also used as a sterilizing agent for medical equipment and supplies. One of the major applications of ethylene oxide is in sterilization processes. Due to its ability to penetrate materials and kill a wide range of microorganisms, it is commonly used to sterilize heat- or moisture-sensitive medical devices, such as surgical instruments, syringes, and catheters.
Vinyl alcohol, also known as ethenol, is an unstable molecule that is rarely isolated in pure form due to its tendency to undergo tautomerization, converting into its more stable tautomer, acetaldehyde. Vinyl alcohol is an important intermediate in various chemical reactions and is of interest in the field of organic chemistry. It is formed as an intermediate during the hydrolysis of vinyl acetate, a common chemical used in the production of polyvinyl acetate (PVA) and polyvinyl alcohol (PVOH).
Method 1: Step method to draw the Lewis dot structure of C2H4O.
In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Here is a little flow chart of how we are going to do this:
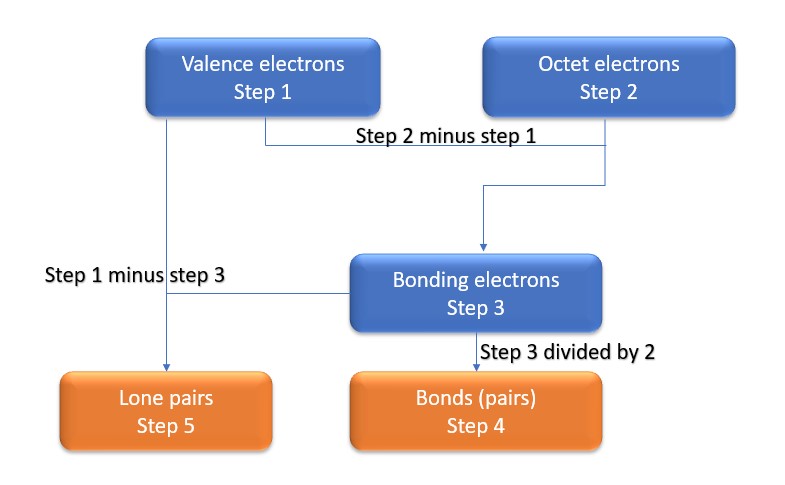
We will go through the steps below, but one thing to note here is that all the valence electrons (step 1) are either lone pairs OR bonding electrons. In other words…. Lone Pairs (Step 5) + Bonding electrons (Step 3) = Valence electrons (Step 1) . Let’s go through this example so we can see this a little more clearly.
Step 1: Find valence electrons for all atoms. This is determined by looking at which column on the periodic table the atom is in, ignoring the transition metals in the middle. Add the valence electrons for each atom together.
C : 2×4 = 8
H : 4×1 = 4
O : 1×6 = 6
Total = 18 valence electrons
Step 2: Find octet electrons for each atom and add them together. Most atoms like 8 electrons to form an octet, however H is an exception to this.
C: 2×8 =16
H: 4×2 = 8
O: 1×8 = 8
Total = 32 “octet” electrons
Step 3: Find the number of bonding electrons. Subtract the valence electrons (step 1) from the octet electrons (step 2). This gives the number of bonding electrons.
32-18=14 bonding electrons.
Step 4: Find number of bonds by diving the number of bonding electrons (step 3) by 2 because each bond is made of 2 e-
14 bonding electrons/2 = 7 bond pairs
Step 5: Find the number of nonbonding (lone pairs) electrons. Subtract bonding electrons (step 3) number from valence electrons (step 1).
18 valence -14 bonding = 4 electrons = 2 lone pair
Now, use the information from step 4 and 5 to draw the Lewis structures. Remembering too (this is important):
Neutral carbon has four bonds and no lone pairs
Hydrogen has one bond and no lone pairs
Neutral oxygen has two bonds and two lone pairs.
[Note: For more information on the natural state of common atoms, see the linked post here.]
The two carbon atoms are the central atoms and go side by side. Now, arrange hydrogens and the oxygen atom on the sides of carbon. The following three Lewis structures are possible for C2H4O:
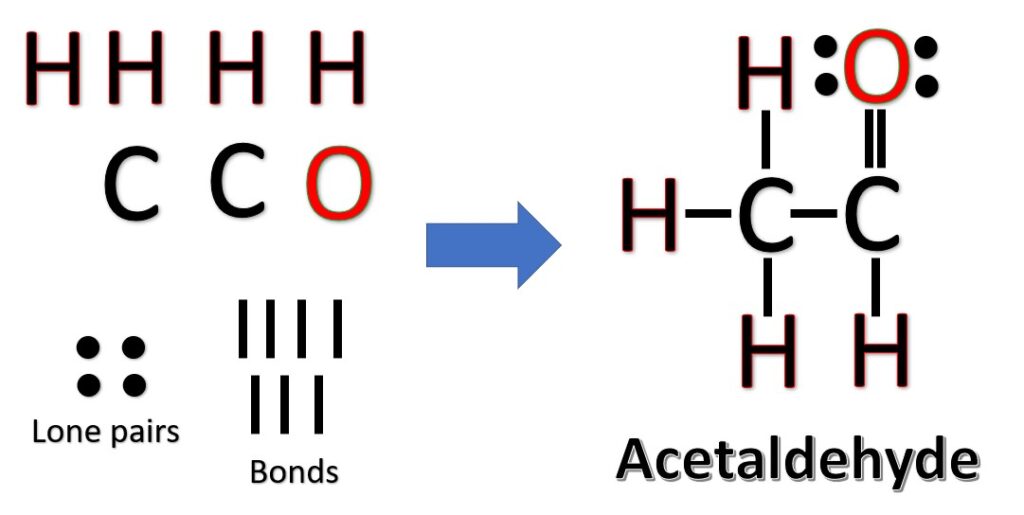
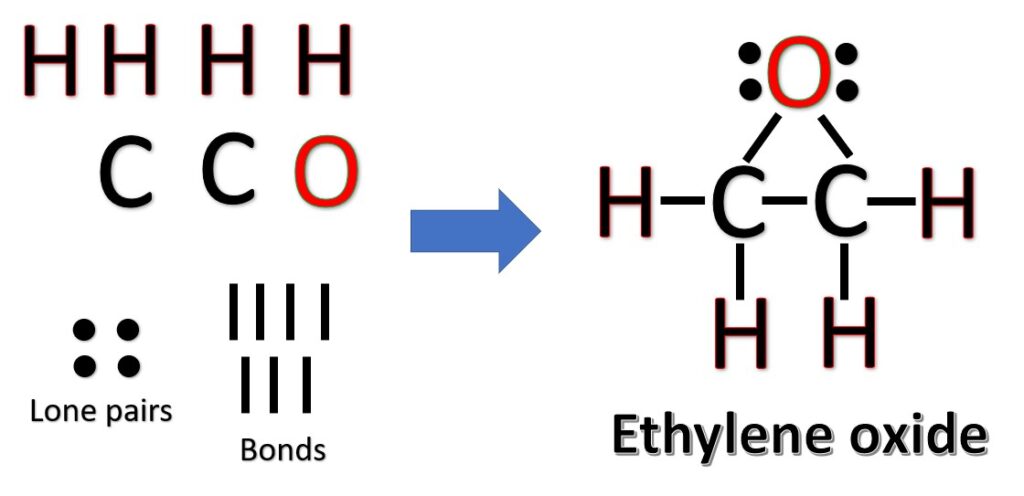

Another (easier) method to determine the structure of C2H4O:
Alternatively a dot method can be used to draw the Lewis structure.
Calculate the total valence electrons in the molecule.
C : 2×4 = 8
H : 4×1 = 4
O : 1×6 = 6
Total = 18 valence electrons
Now, treat the atoms and electrons like puzzle pieces. Put carbons in the center and arrange hydrogen and oxygen atoms on the sides. Remember the natural state of each atom, as discussed above. [Oxygen: 2 bonds, 2 LP; Hydrogen: 1 bonds, 0 LP; Carbon: 4 bonds, 0 LP] Also, make sure sure each atom has an octet, which in the case of hydrogen is only two electrons.
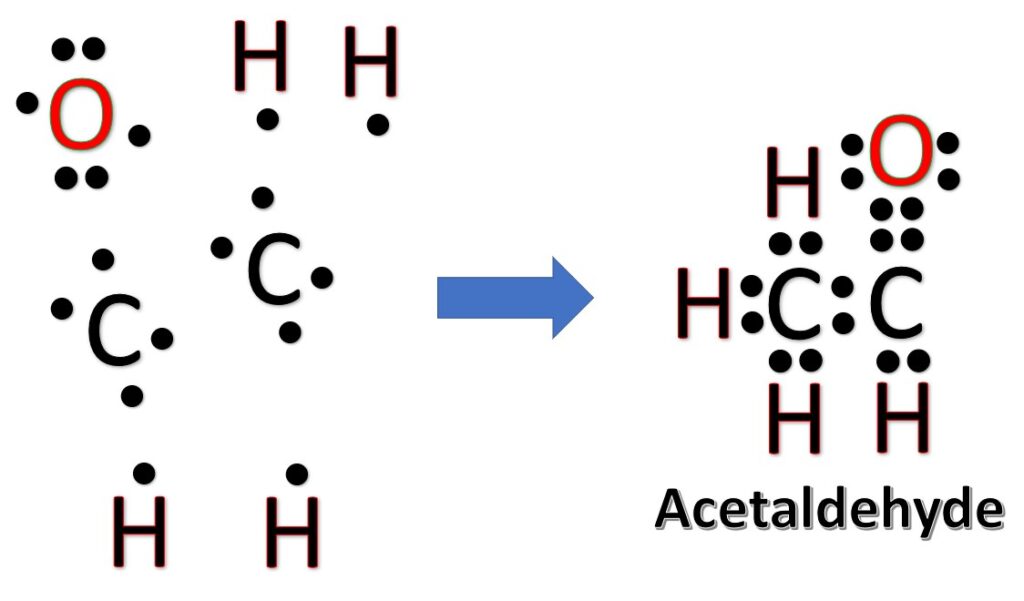
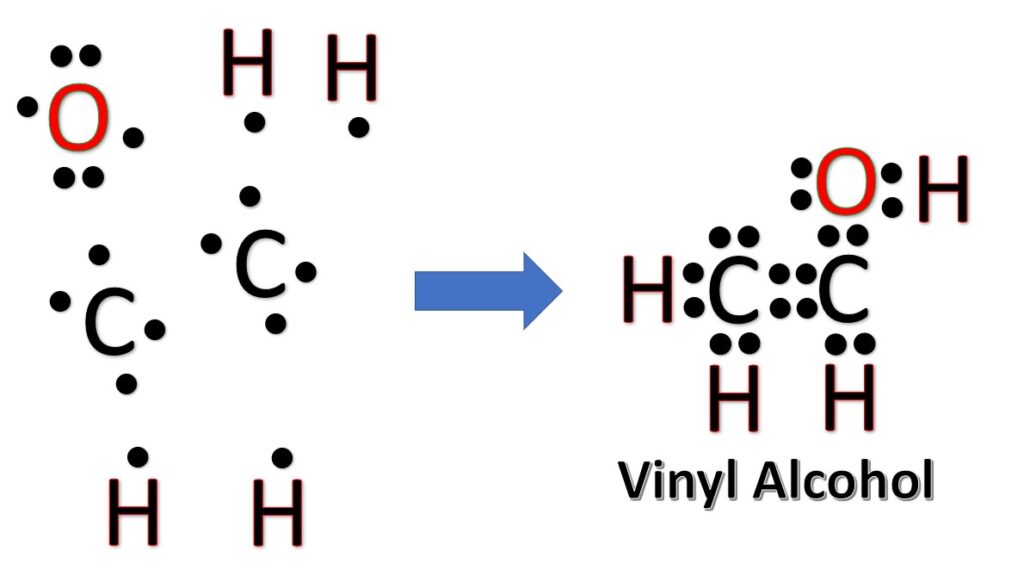
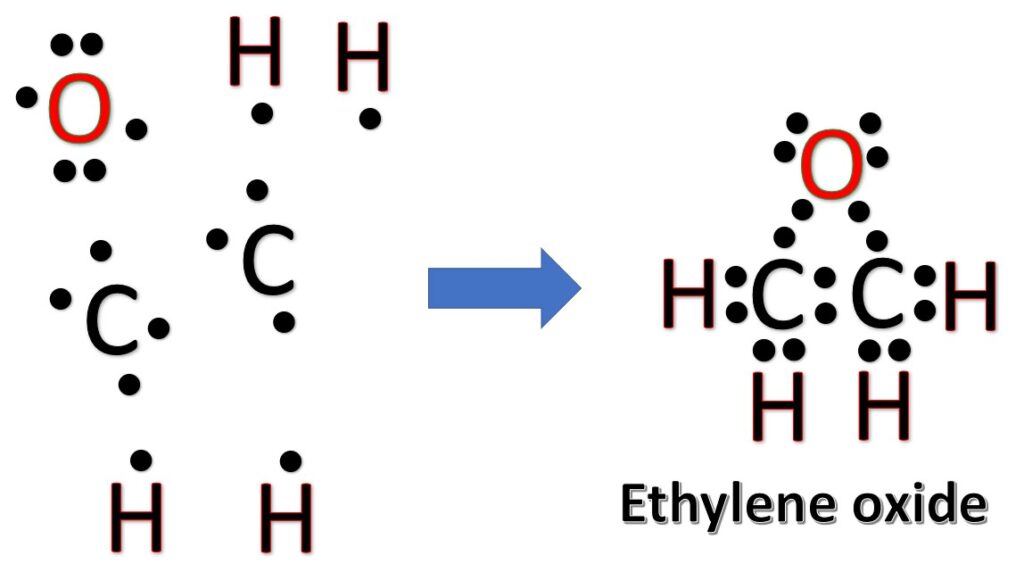
Frequently asked questions:
Q: So what is the difference between the two methods?
A: In the first method, we are figuring out all of the lone pairs and bonds first, then placing those electrons and bonds on the atoms to form a molecule. In the puzzle method, we already have lone pairs and bonding electrons assigned to each atom, so all we need to do is push puzzle pieces together to get a molecule. In each method, we need to remember the “happy state” of each atom, ei hydrogen likes 1 bond and no lone pairs, uncharged carbon likes four bonds and no lone pairs ect.
Q: How is it that we can get three different molecules from the same formula?
A: It is all in how you arrange the atoms. As long as you are following the rules and placing the appropriate number of electrons on each atom, there may be several correct answers. BTW, when you have different molecules with the same formula, they are called constitutional isomers. Here is a good post about constitutional isomers.
Q: OK, so which one is right?
A: That depends on which Lewis Structure of C2H4O your professor is looking for. Any of the three are technically correct, so if your professor did not give any guidance (ei a name of a compound), all should get you full credit.
And now some video:
This is a quick video we put together that visually demonstrates the two methods for Lewis structure and Lewis dot problems.
And finally, the Lewis structure study guide:
Here it is, this is our one-page guide to Lewis Dot and Lewis Structures:

