What is the Lewis Structure of ClO4–?
The Lewis Structure of ClO4– is an ionic structure which has chlorine as the central atom connected to four oxygen atoms. Three of the four oxygen atoms are double bonded to chlorine and are neutral, while one chlorine atom is single bonded to chlorine and is negatively charged. It has a tetrahedral geometry.
ClO4–, also known as the perchlorate ion, is a highly oxidizing and reactive ion that can be synthesized through various methods, including the reaction of chlorine with sodium hydroxide. Perchlorate ions are used in a variety of industrial processes, including as rocket propellants, fireworks and explosives. They are considered to be environmental pollutants as they are quite persistent after dispersion. Unfortunately, they can be found in drinking water, soil, and various food products. Perchlorate ions are believed to have harmful effects on human beings, as they can interfere with the proper functioning of the thyroid gland by inhibiting the uptake of iodide, which is necessary for the production of thyroid hormones. This can lead to a condition called hypothyroidism, which can cause developmental delays in children and other health problems in adults.
Method to draw the Lewis dot structure of perchlorate ion, ClO4–.
Here is a little flow chart of how we are going to do this:

We will go through the steps below, but one thing to note here is that the valence electrons (step 1) are either lone pairs OR bonding electrons. In other words…. Lone Pairs (Step 5) + Bonding electrons (Step 3) = Valence electrons (Step 1) . Let’s go through this example so we can see this a little more clearly.
Step 1: Find valence electrons for all atoms. This is determined by looking at which column on the periodic table the atom is in, ignoring the transition metals in the middle. Add the valence electrons for each atom together. (we will use the shorthand of “e-” to symbolize the word electron)

Cl has 7 valence electrons. When we picture it like this, it becomes easier to see that stable and uncharged chlorine likes to have one bond and three lone pairs of electrons.

O: 6 x 4 atoms =24 total valance electrons. When we picture it like this, it becomes easier to see that stable and uncharged oxygen likes to have two bonds and two lone pairs of electrons.
Total = 31+1 = 32*
* add an electron to the total since the molecule has -1 charge on it.
Step 2: Find octet e- for each atom and add them together.
Cl : 8
O = 8×4 = 32
Total = 40
Step 3: Find the bonding e-. These are the electrons that comprise the bonds of the atom. Subtract step 1 total from step 2.
40 – 32 = 8e- bonding electrons
Step 4: Find number of bonds by diving the number in step 3 by 2 (because each bond is made of 2 e-)
8e- / 2= 4 bond pairs
Step 5: Find the number of nonbonding (lone pairs) e-. Subtract step 3 number from step 1.
32 – 8 = 24e- = 12 lone pair
Use information from step 4 and 5 to draw the Lewis structure.

HOWEVER, this structure above is not the right one! While every atom has an octet, and it follows our steps properly, it does not give you the right answer because this molecule has a charge of -4, which is not what perchlorate has. Therefore, we have to move some lone pairs around to get to a charge of -1.

In the structure above, we have moved three lone pairs and turned them into bonding electrons, which puts double bonds on three of the four oxygen atoms. While this may not look right, because Cl has more than an octet, it is the correct structure of ClO4–.
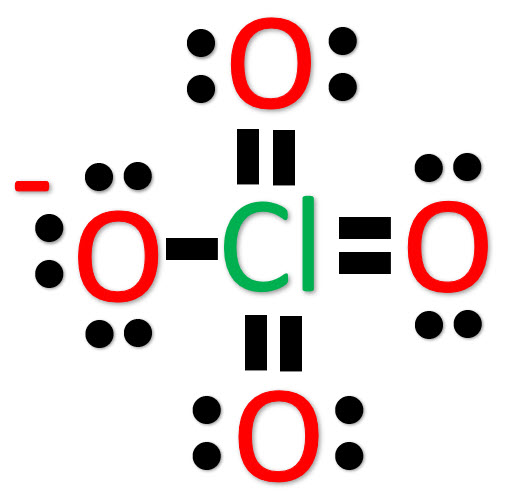
Another (easier) method to determine the structure of ClO4–
Alternatively a dot method can be used to draw the Lewis structure.
Calculate the total valence electrons in the molecule.
Cl : 7
O : 6×4 = 24
Total = 31+1 = 32*
Treat the atoms and electrons like puzzle pieces. Put chlorine in center and arrange oxygen atoms on the sides. put a pair of electrons connecting the chlorine atom and the remaining electrons on oxygen atoms.


Frequently Asked Questions:
Q: So what is the difference between the two methods to find the Lewis Structure of ClO4–?
A: In the first method, we are figuring out all of the lone pairs and bonds first, then placing those electrons and bonds on the atoms to form a molecule. In the puzzle method, we already have lone pairs and bonding electrons assigned to each atom, so all we need to do is push puzzle pieces together to get a molecule. In each method, we need to remember the “happy state” of each atom, ei hydrogen likes 1 bond and no lone pairs, uncharged carbon likes four bonds and no lone pairs ect.
Q: Why does chlorine appear to have 14 electrons around it?
A: In some cases, chlorine can take on more valence electrons than in an octet, this is referred to as hypervalence. Generally, this indicates the ion will be highly reactive.
Q: How does resonance affect this ion?
A: Any one of the oxygen atoms can be negatively charged at any one time. Another way to think about this is that the negative charge/single bond rotates quickly between the four oxygen atoms, essentially equally distributing the negative charge over all four.
And now some video:
This is a quick video we put together that visually demonstrates the two methods for Lewis structure and Lewis dot problems.
And finally, the Lewis Structure study guide:
Here it is, this is our one-page guide to Lewis Dot and Lewis Structures:

