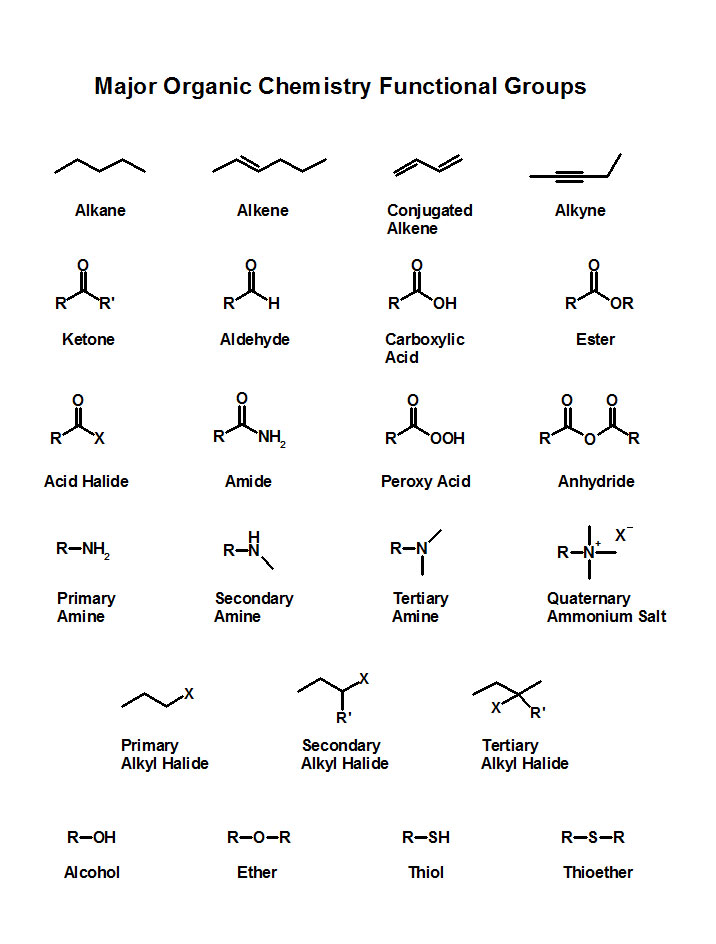
Functional Groups in Organic Chemistry
Welcome back. Let’s not beat around the bush on this one: functional groups in organic chemistry are why we can do any organic chemistry in the first place. Functional groups are the basis of why molecules can and will react with each other. Without functional groups, everything would be straight chain alkanes and other boring hydrocarbons. So it’s important to learn functional groups, and how they will interact with nucleophiles and electrophiles to react to form new organic molecules.
Major Disclaimer: This is not meant to be a comprehensive review of all of the functional groups out there, however it’ll be a nice start and a good reference for you.
Hopefully you understand why they are important, now we just have to determine what some of the different types are.
What to learn about nucleophiles? Click on the link to check it out
Hydrocarbons: these are simply composed of carbon and hydrogen. This group is alkanes, cycloalkanes, alkenes, and alkynes. Don’t forget about conjugated alkenes too, as they are important in many organic processes such as the Diels-Alder reaction. While alkanes and cycloalkanes are not particularly reactive, alkenes and alkynes definitely are.
Carbonyls: a “carbon double bond oxygen” is a carbonyl. It is one of the more important electrophiles you will see in this course. While there are different variations which can make the carbonyl more or less reactive, the basic functional group is still the same. The important point here is to know which types of carbonyls are more electrophilic and which ones are less. Generally speaking, if there is an electron withdrawing group attached to the carbonyl carbon, that carbonyl will be more electrophilic and more reactive.
Alkyl Halides: alkanes which are connected to a halogen atom (F, Cl, I, and Br) are good electrophiles. These can participate in nucleophilic substitution reactions and elimination reactions. They reactivity depends on the type of alkyl halide (F, Cl, I, Br), its substitution (primary, secondary, tertiary) and the desired reaction (SN1, SN2, E1, E2).
Alcohols, Amines, and Thiols: these are generally very good nucleophiles, as the heteroatoms have lone pairs which will attack an electrophile.
Ethers: do not undergo many organic reactions themselves, but sometimes can be the product of a reaction. Some chemists refer to ethers as “dead molecules” because of their low reactivity.
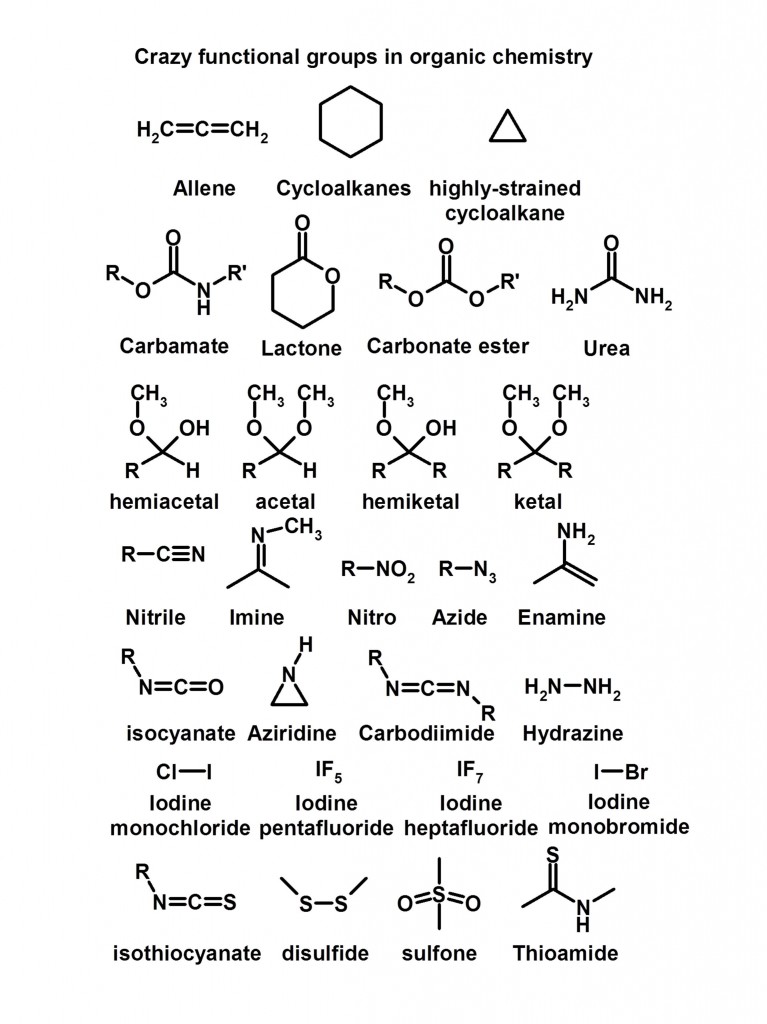
And now for some crazy functional groups….