What is a cell?
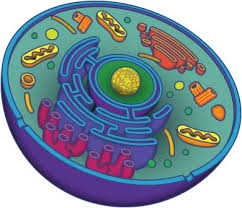
What is a cell? Cells are considered the basic units of life in part because they come in discrete and easily recognizable packages. All living organisms consist of cells. You are made of billions and billions of cells.
Whether an organisms contains 1 cell or 1,000,000,000 cells, they provide life to all creatures. There are different jobs and purposes for each different cell in complex living beings, and there are unique types of cells.
The purpose of this webpage is to bring clarity to the different types of organelles and structures contained within an animal cell.
Cells are divided up into 2 different types: 1) prokaryotic & 2) eukaryotic. Eukaryotic cells are distinguished by the fact that they have nuclei. In general, there is 1 (and only 1) nucleus in every eukaryotic cell, and that nucleus contains the genetic material for the cell in the form of chromatin.
We won’t talk much about prokaryotic cells here but they tend to be smaller in size and volume. Although their size does vary, they range between 1-10 millimeters in diameter. Instead of reproducing sexually, they reproduce by fission, meaning that each offspring contains the same genetic information as their parent cell. Some think of the fission process as the cell just dividing itself in half. Prokaryotic cells are enclosed by a cell wall made of many sugar molecules hooked to each other in a complex fashion.
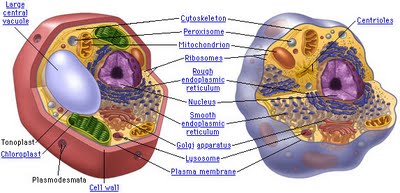
The Eukaryotic cell:


Like a prokaryotic cell, a eukaryotic cell has a plasma membrane, cytoplasm, and ribosomes. However, unlike prokaryotic cells, eukaryotic cells have a membrane-bound nucleus, numerous membrane-bound organelles (including the endoplasmic reticulum, Golgi apparatus, chloroplasts, and mitochondria) and several rod-shaped chromosomes.
When we talk about the cell, we are going to start from the inside and work our way out.
The nucleus:
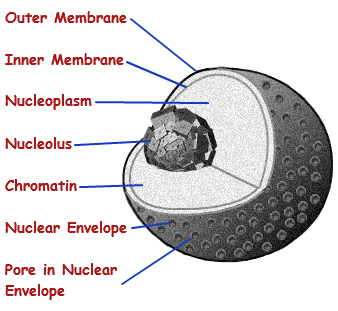
So what is the cell and why is the nucleus important? The nucleus is a membrane enclosed organelle. It has almost all of the cell’s genetic material (DNA) with a large variety of proteins to form chromosomes. The genes within these chromosomes are the cell’s nuclear genome. The function of the nucleus is to maintain the integrity of these genes and to control the activities of the cell by regulating gene expression — the nucleus is therefore the control center of the cell. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and separates its contents from the cellular cytoplasm, and the nuclear lamina, a meshwork within the nucleus that adds mechanical support, much like the cytoskeleton supports the cell as a whole. Because the nuclear membrane is impermeable to most molecules, nuclear pores are required to allow movement of molecules across the envelope. These pores cross both of the membranes, providing a channel that allows free movement of small molecules and ions. The movement of larger molecules such as proteins is carefully controlled, and requires active transport regulated by carrier proteins. Nuclear transport is crucial to cell function, as movement through the pores is required for both gene expression and chromosomal maintenance.
Although the interior of the nucleus does not contain any membrane-bound subcompartments, its contents are not uniform, and a number of subnuclear bodies exist, made up of unique proteins, RNA molecules, and particular parts of the chromosomes. The best known of these is the nucleolus, which is mainly involved in the assembly of ribosomes. After being produced in the nucleolus, ribosomes are exported to the cytoplasm where they translate mRNA.
Inside the nucleus are:
1) Chromosomes: store and organize genes that allow cell division.
2) Nuclear pores: transport regulatory factos and gene products.
3) Messenger ribonucleic acid (mRNA): produce messages that code for proteins.
4) Nucleolus: produce ribosomes that functioning in the expression of gene code into proteins.
What is a cell? The Endoplasmic Reticulum
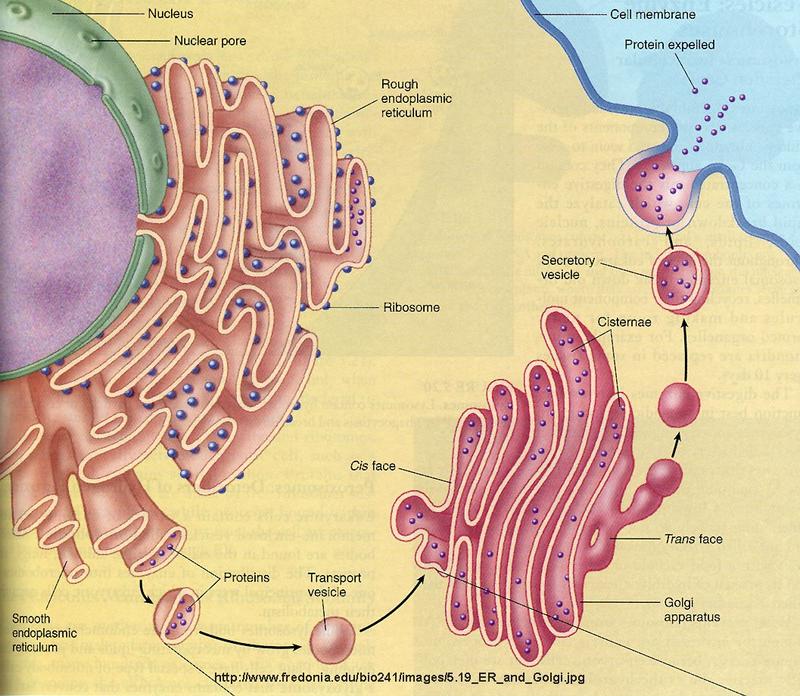
If we think of a cell as a “bunch of membranes”, the endoplasmic reticulum absolutely would be the most prominent of them all. One membrane to rule them all.
Almost half of the total membrane surface area in an animal cell comes from the endoplasmic reticulum (ER). The organelle called ‘endoplasmic reticulum’ is an essential manufacturing site for lipids (fats) and many proteins. Much of what is synthesized is made for and goes to the other parts of the cell.
There are two types of endoplasmic reticulum: rough endoplasmic reticulum (rough ER) and smooth endoplasmic reticulum (smooth ER). Even though these are separate organelles, which are not physically connected to each other, they provide much of the same function. Cells concentrating on the production of proteins usually have a larger amount of rough ER while cells producing lipids (fats) & steroid hormones will have more smooth ER.
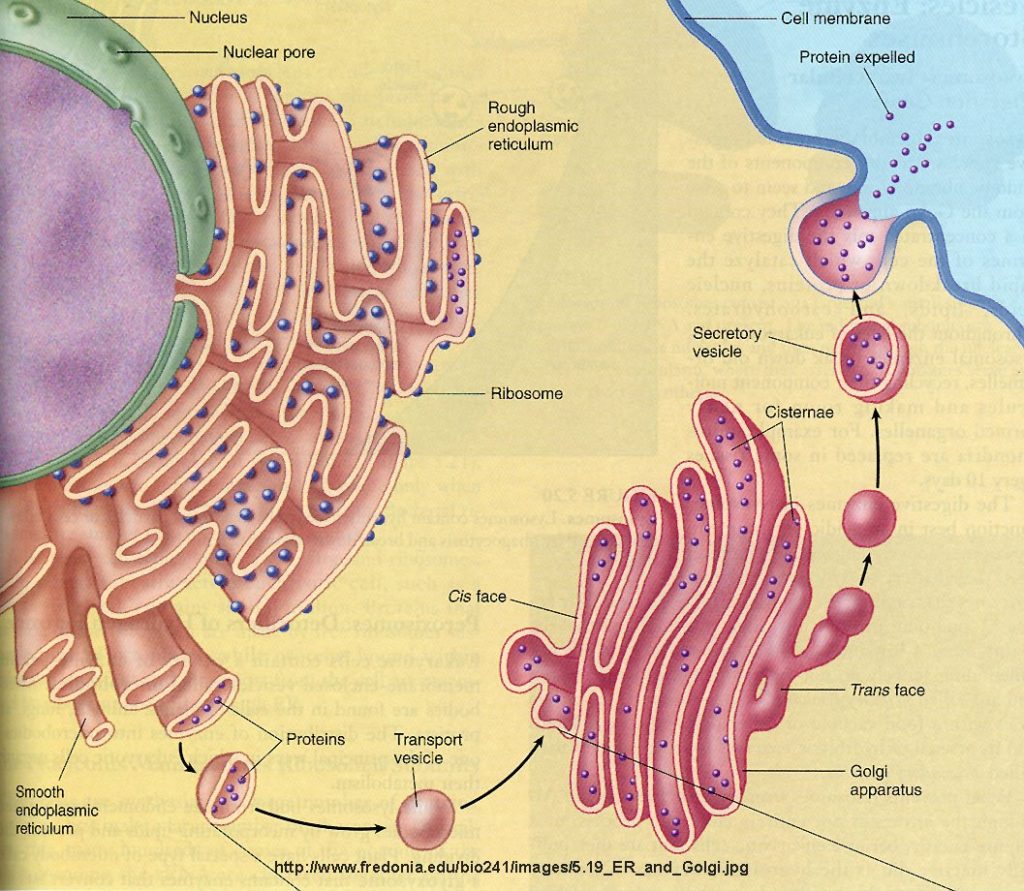
Part of the rough ER is connected to the with nucleus via the nuclear envelope. The Golgi apparatus is also closely correlated with the ER and recent observations suggest that parts of the two organelles, i.e. the ER and the Golgi complex, are so close that some chemical products probably pass directly between them instead of being packaged into vesicles (droplets enclosed within a membrane) and transported to them through the cytoplasm.
ROUGH ENDOPLASMIC RETICULUM
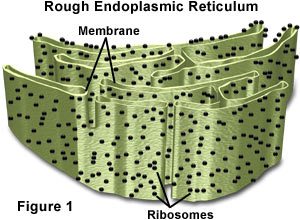
The Rough ER is an extensive organelle composed of a greatly convoluted but flattish sealed sac that is continuous with the nuclear membrane. It is called ‘rough’ endoplasmic reticulum because it is studded on its outer surface (the surface in contact with the cytosol) with ribosomes. These are called membrane bound ribosomes and are firmly attached to the outer cytosolic side of the ER. There are over 13,000,000 ribosomes present on the RER in the average liver cell. Rough ER is found throughout the cell but the density is higher near the nucleus and the Golgi apparatus. This absolutely should be a clue about its function.
Ribosomes on the Rough ER are called ‘membrane bound’ and are responsible for the assembly of many proteins. This process is called translation, and involves coupling amino acids to create proteins. Certain cells of the pancreas and digestive tract produce a large volume of protein as enzymes. Many of the proteins are produced in high volume in the cells of the pancreas and the digestive tract and function as digestive enzymes.
The rough ER working with membrane bound ribosomes takes polypeptides and amino acids from the cytosol and continues protein assembly including, at an early stage, recognizing a ‘destination label’ attached to each of them. Proteins are produced for the plasma membrane, Golgi apparatus, secretory vesicles, plant vacuoles, lysosomes, endosomes and the endoplasmic reticulum itself. Numerous proteins are delivered into the lumen or space inside the ER whilst others are processed within the ER membrane itself. In the lumen some proteins have sugar groups added to them to form glycoproteins. Some have metal groups added to them. It is in the rough ER for example that 4 polypeptide chains are bound to form hemoglobin.
Protein folding unit
It is in the lumen of the rough ER that proteins are folded to produce the highly important biochemical architecture which will provide ‘lock and key’ and other recognition and linking sites. This is essential to the protein’s function. When a protein loses this architecture, it is said to be denatured, a process that can happen by heating, among other ways.
Protein quality control section
It is also in the lumen that an amazing process of quality control checking is carried out. Proteins are subjected to a quality control check and any that are found to be incorrectly formed or incorrectly folded are rejected. These rejects are stored in the lumen or sent for recycling for eventual breakdown to amino acids, which can then be reused or flushed through the liver. A type of emphysema (a lung disease often caused by smoking) is caused by the ER quality control section continually rejecting an incorrectly folded protein. The protein is wrongly folded as a result of receiving an altered genetic message. The required protein is never exported from the lumen of rough ER. Research into protein structure failures relating to HIV are also focusing on reactions in the ER.
From Rough ER to Golgi
In most cases proteins are transferred to the Golgi apparatus for ‘finishing’. They are conveyed in vesicles or possibly directly between the ER and Golgi surfaces. After ‘finishing’ they are delivered to specific locations for use as enzymes or cell signals.
SMOOTH ENDOPLASMIC RETICULUM

Smooth ER (SER – not to be confused with Serine, an amino acid whose 3-letter designation is Ser) is much more tubular than rough ER and forms a separate closed interconnecting network. It is found almost exclusively evenly dispersed throughout the cell’s cytoplasm.
There are no ribosomes studding the outside, therefore it is referred to as the Smooth ER.
Smooth ER is devoted almost exclusively to the manufacture of lipids (fats and fatty acids) and in some cases to the metabolism of them and accompanying products. In cells found in the liver for example, smooth ER enables glycogen that is stored as granules on the external surface of smooth ER to be broken down to glucose. Smooth ER is also involved in the production of steroid hormones in the adrenal cortex and endocrine glands.
Smooth ER – the detoxification place.
Hospitals and in-treatment facilities are not the only places your body can detox. Smooth ER also plays a large part in detoxifying a number of organic chemicals converting them to safer water-soluble products, which can then be flushed through the kidneys or the liver.
Large amounts of smooth ER are found in liver cells where one of its main functions is to detoxify products of natural metabolism and to attempt to detoxify massive amounts of ethanol derived from excess alcoholic drinking that some (mostly college freshman) engage in. To assist with this, smooth ER can double its surface area within a few days, returning to its normal size when this toxic assault has dies down. If the Smooth ER is not allowed to return to normal size, alcohol tolerance can ensue.
The Golgi apparatus, the next stop.
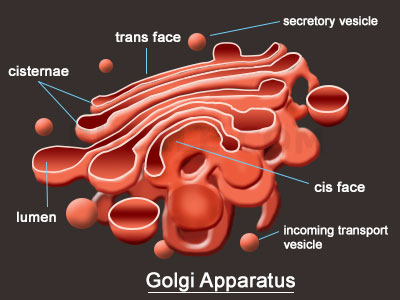
The Golgi apparatus functions as a molecular assembly line in which membrane proteins undergo extensive post-translational modification. Many Golgi reactions involve the addition of sugar residues to membrane proteins and secreted proteins. The carbohydrates that the Golgi attaches to membrane proteins are often quite complex, and their synthesis requires multiple steps.
In electron micrographs, the Golgi apparatus looks like a set of flattened sacs, which many people say look like a stack of pancakes. Vesicles that bud off from the endoplasmic reticulum fuse with and or absorbed by the closest Golgi membranes, called the cis-Golgi. Molecules then travel through the Golgi via vesicle transport until they reach the end of the assembly line at the farthest sacs from the ER — called the trans-Golgi. At each workstation along the assembly line, Golgi enzymes catalyze distinct reactions. Later, as vesicles of membrane lipids and proteins bud off from the trans-Golgi, they are directed to their appropriate destinations — either lysosomes, storage vesicles, or the plasma membrane.
Vesicles: The same type of bubble does all sorts of different things.
In cell biology, vesicles are miniscule membrane-enclosed sacs within the cell organelles of eukaryotic cells. These sacs help transport or absorb proteins, enzymes and other cell necessities. Inside the membrane sac of a vesicle are macromolecules that require the ability to move beyond cell walls. The membrane encompassing the sac fuses with the outer cell wall to allow these macromolecules to pass through. Vesicles are important parts of human cells, although they also appear in other multicellular organisms.
Eukaryotic cells are the only cells to have vesicles. These cells are a specific type of cell in which various cell organelles are contained separately inside membranes. The cell organelles of animal cells require a transportation system in order to exchange essential materials. Depending on the type of cell, vesicles transport proteins or enzymes, absorb food cells, store and release neurotransmitters or perform a number of other functions for organelles. The cell type and purpose determine the specific function of a vesicle.
Human, plant and animal cells use a variety of types of vesicles, depending on the type of cell and its specific intended function. For example, lysosomes are a type of vesicle needed for digestion. Lysosomes hold enzymes needed to break down food cells. As the food is absorbed, a lysosome vesicle bonds to the vesicle holding the food, releasing its enzymes through a process called phagocytosis. These enzymes break down food cells into smaller parts for absorption by other cells.
Secretory vesicles are commonly associated with nerve cells in a human or animal. These membranes hold neurotransmitters. The nervous system triggers these components through hormonal signals. Through the process of exocytosis, the secretory vesicle’s outer membrane fuses to the nerve terminal, releasing neurotransmitters into the space between nerve endings known as the synaptic cleft. Neurotransmitters carry information from one nerve ending to the next, traveling along the central nervous system to the brain.
As internal cell mechanisms, vesicles perform transportation, absorption and storage functions imperative to numerous bodily functions. Without these miniscule membrane sacs, cells would be unable to exchange materials needed to maintain healthy cell development and crucial system processes. In short, without vesicles, human and other multicellular organisms could not exist, because the crucial chemical cell processes needed would have no method by which to exchange essential materials.
Centrioles
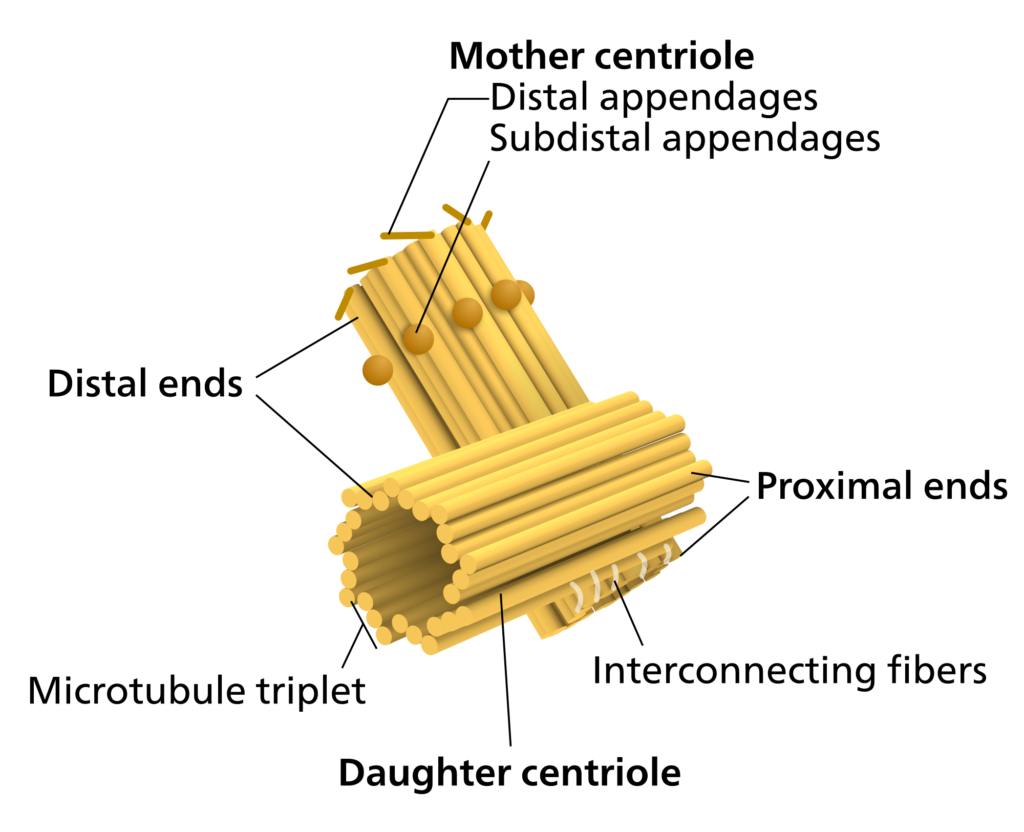
All animal cells have two small organelles known as centrioles. The centrioles help the cell to divide through the process of mitosis and meiosis. The centrioles together are usually located near the nucleus in the centrosome. Centrosomes is granular mass that is the organizing center for the microtubules. The position of the centrosome within the centrosome is at right angles to each other. Centrioles are made of nine bundles of microtubules, that are arranged in a ring.
The centrioles play a major role in cell division. In animal cells the centrioles play a major role in cell division but the plant cells have the ability to reproduce even without the centrioles. In certain animal cells, like the female oocytes, some cells have have shown successful division even in the absence of the centrioles. The absence of centrioles causes divisional errors and delays in the mitotic process. It is consequently suggested that centrioles have evolved as a cell refinement, that makes mitosis a more efficient and lesser error prone process.
Found in most eukaryotic cells, the centrioles are made of groups of microtubules which are arranged in a pattern of 9+3. The pattern of the microtubules for a ring of 9 microtubules known as “triplets” and the microtubules are arranged at right angles to one another. In animals cells, the centrioles help the organizing and assembly of microtubules during the process of cell division. The replication of centrioles happens in the interphase of mitosis and meiosis.
At the anaphase and telophase stages, the centrioles appear as two cylindrical structures. They are open at both the ends and are located at right angles to each other. Each of the centriole is made up of nine triplet fibers and these triplet fibers are arranged in a circular manner that gives it a barrel-shaped appearance. Within the centrosome a mother centriole and the daughter centriole are arranged at perpendicular angles to each other. These two centrioles are tightly attached to each other and are surrounded by dense matrix called pericentriolar material. The mother centriole is a mature structure, as it has additional appendages and is involved in anchoring and positioning of the microtubules. Comparatively the daughter is a young and immature structure. The length of the centriole is about 3,000 to 5,000 Angstroms and it is about 1,500 to 1,800 Angstroms in diameter. The triplets are tilted, they form an angle of 40 degrees to the radius of the cylinder. Each triplet fiber is composed of three sub-tubules or sub-fibres. Each sub-tubule is of 250 Angstroms in diameter. These sub-tubules are hollow structures and their walls are made of monomeric units of proteins.The sub-tubules are made up of protein tubulin. The centriole internally shows a characteristic cart wheel structure. The cart wheel structure has a prominent central rod, and nine spokes radiating from the central rod. In 1958 scientists Bellis et al., said that the centrioles are surrounded by two crowns that lie one above the other. Each crown like structure is comprised of nine spheres that are called as massules or corpuscles.All the structures that surrounds the centriole together constitute the centriole satellite. The number of these satellites varies. The centrosome structure is made of lipids and proteins. However, it also contains carbohydrates and nucleic acids too.
Mitochondria
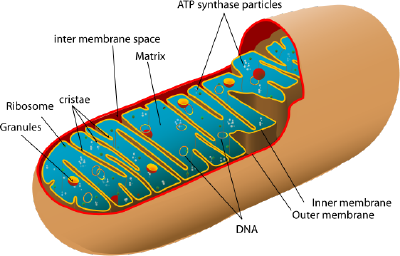

Mitochondria are tiny organelles inside cells that are involved in releasing energy from food and fat. This process is known as cellular respiration. It is for this reason that mitochondria are often referred to as the powerhouses of the cell. Cells that need a lot of energy, like muscle cells, can contain thousands of mitochondria.
When the breakdown products from the digestion of food find their way into the cell, a series of chemical reactions occur in the cytoplasm. This lets some of the energy locked up in these products to be released and incorporated into the universal energy supplier in cells known as ATP (adenosine triphosphate).
The remaining molecular fragments from the process then enter the mitochondria, and in a complex series of steps, they are finally converted into carbon dioxide and water. The energy locked up in these fragments is incorporated into more ATP.
The ATP molecules produced in this way can then be used by the cell to supply the energy needed to function. ATP → ADP + P + energy to function..
Cytosol
The cytoplasm is a thick, gelatinous, semitransparent fluid present in both eukaryotic and prokaryotic cells. It is bounded by the plasma membrane. In eukaryotes, the nucleus of a cell is separated from the cytoplasm by a double membrane, known as nuclear membrane.
The cytoplasm has three major components – the cytosol, organelles, and cytoplasmic inclusions, of which the cytosol is the thick fluid where other cytoplasmic elements remain suspended. The cytosol consists of 70% to 90% water, and it is usually colorless.
Structure of Cytoplasm
As mentioned already, the three main elements of the cytoplasm are, cytosol, organelles, and cytoplasmic inclusions. Here is a brief discussion about their structure and functions.
The cytosol is the part of cytoplasm that is not occupied by any organelle. It is a gelatinous fluid, where other components of the cytoplasm remain suspended. It mainly consists of cytoskeleton filaments, organic molecules, salt, and water. Cytoskeleton filaments are the protein filaments. The cytoskeleton consists of structures called ‘microfilaments’ and ‘microtubules’ that form a skeletal network, thereby giving shape to the cell and holding the various organelles in place.
Microfilaments are thin fibers made up of actin polymers. They facilitate the movement of substances inside a cell. Microtubules are hollow cylindrical structures made up of tubulin polymers. They assist the movement of different organelles, and play a crucial role in cell division by aiding the movement of chromosomes in the nucleus during mitosis. The cytosol also contains enzymes, fatty acids, sugar, and amino acids. The cytosol accounts for almost 70% of the total cell volume.
The jelly-like fluid that fills a cell is called cytoplasm. It is made up of mostly water and salt. Cytoplasm is present within the cell membrane of all cell types and contains all organelles and cell parts. Cytoplasm has various functions in the cell.
Most of the important activities of the cell occur in the cytoplasm. Cytoplasm contains molecules such as enzymes which are responsible for breaking down waste and also aid in metabolic activity.
Cytoplasm is responsible for giving a cell its shape. It helps to fill out the cell and keeps organelles in their place. Without cytoplasm, the cell would be deflated and materials would not be able to pass easily from one organelle to another.
Plasma membrane
Like all other cellular membranes, the plasma membrane consists of both lipids and proteins. The fundamental structure of the membrane is the phospholipid bilayer, which forms a stable barrier between two aqueous compartments. In the case of the plasma membrane, these compartments are the inside and the outside of the cell. Proteins embedded within the phospholipid bilayer carry out the specific functions of the plasma membrane, including selective transport of molecules and cell-cell recognition.

The Phospholipid Bilayer
The plasma membrane is the most thoroughly studied of all cell membranes, and it is largely through investigations of the plasma membrane that our current concepts of membrane structure have evolved. The plasma membranes of mammalian red blood cells (erythrocytes) have been particularly useful as a model for studies of membrane structure. Mammalian red blood cells do not contain nuclei or internal membranes, so they represent a source from which pure plasma membranes can be easily isolated for biochemical analysis. Indeed, studies of the red blood cell plasma membrane provided the first evidence that biological membranes consist of lipid bilayers. In 1925, two Dutch scientists (E. Gorter and R. Grendel) extracted the membrane lipids from a known number of red blood cells, corresponding to a known surface area of plasma membrane. They then determined the surface area occupied by a monolayer of the extracted lipid spread out at an air-water interface. The surface area of the lipid monolayer turned out to be twice that occupied by the erythrocyte plasma membranes, leading to the conclusion that the membranes consisted of lipid bilayers rather than monolayers.
The bilayer structure of the erythrocyte plasma membrane is clearly evident in high-magnification electron micrographs . The plasma membrane appears as two dense lines separated by an intervening space—a morphology frequently referred to as a “railroad track” appearance. This image results from the binding of the electron-dense heavy metals used as stains in transmission electron microscopy to the polar head groups of the phospholipids, which therefore appear as dark lines. These dense lines are separated by the lightly stained interior portion of the membrane, which contains the hydrophobic fatty acid chains.
The plasma membranes of animal cells contain four major phospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin), which together account for more than half of the lipid in most membranes. These phospholipids are asymmetrically distributed between the two halves of the membrane bilayer. The outer leaflet of the plasma membrane consists mainly of phosphatidylcholine and sphingomyelin, whereas phosphatidylethanolamine and phosphatidylserine are the predominant phospholipids of the inner leaflet. A fifth phospholipid, phosphatidylinositol, is also localized to the inner half of the plasma membrane. Although phosphatidylinositol is a quantitatively minor membrane component, it plays an important role in cell signaling, as discussed in the next chapter. The head groups of both phosphatidylserine and phosphatidylinositol are negatively charged, so their predominance in the inner leaflet results in a net negative charge on the cytosolic face of the plasma membrane.
In addition to the phospholipids, the plasma membranes of animal cells contain glycolipids and cholesterol. The glycolipids are found exclusively in the outer leaflet of the plasma membrane, with their carbohydrate portions exposed on the cell surface. They are relatively minor membrane components, constituting only about 2% of the lipids of most plasma membranes. Cholesterol, on the other hand, is a major membrane constituent of animal cells, being present in about the same molar amounts as the phospholipids.
Two general features of phospholipid bilayers are critical to membrane function. First, the structure of phospholipids is responsible for the basic function of membranes as barriers between two aqueous compartments. Because the interior of the phospholipid bilayer is occupied by hydrophobic fatty acid chains, the membrane is impermeable to water-soluble molecules, including ions and most biological molecules. Second, bilayers of the naturally occurring phospholipids are viscous fluids, not solids. The fatty acids of most natural phospholipids have one or more double bonds, which introduce kinks into the hydrocarbon chains and make them difficult to pack together. The long hydrocarbon chains of the fatty acids therefore move freely in the interior of the membrane, so the membrane itself is soft and flexible. In addition, both phospholipids and proteins are free to diffuse laterally within the membrane—a property that is critical for many membrane functions.
Because of its rigid ring structure, cholesterol plays a distinct role in membrane structure. Cholesterol will not form a membrane by itself, but inserts into a bilayer of phospholipids with its polar hydroxyl group close to the phospholipid head groups. Depending on the temperature, cholesterol has distinct effects on membrane fluidity. At high temperatures, cholesterol interferes with the movement of the phospholipid fatty acid chains, making the outer part of the membrane less fluid and reducing its permeability to small molecules. At low temperatures, however, cholesterol has the opposite effect: By interfering with interactions between fatty acid chains, cholesterol prevents membranes from freezing and maintains membrane fluidity. Although cholesterol is not present in bacteria, it is an essential component of animal cell plasma membranes. Plant cells also lack cholesterol, but they contain related compounds (sterols) that fulfill a similar function.
Recent studies suggest that not all lipids diffuse freely in the plasma membrane. Instead, discrete membrane domains appear to be enriched in cholesterol and the sphingolipids (sphingomyelin and glycolipids). These clusters of sphingolipids and cholesterol are thought to form “rafts” that move laterally within the plasma membrane and may associate with specific membrane proteins. Although the functions of lipid rafts remain to be understood, they may play important roles in processes such as cell signaling and the uptake of extracellular molecules by endocytosis.
Membrane Proteins
While lipids are the fundamental structural elements of membranes, proteins are responsible for carrying out specific membrane functions. Most plasma membranes consist of approximately 50% lipid and 50% protein by weight, with the carbohydrate portions of glycolipids and glycoproteins constituting 5 to 10% of the membrane mass. Since proteins are much larger than lipids, this percentage corresponds to about one protein molecule per every 50 to 100 molecules of lipid. In 1972, Jonathan Singer and Garth Nicolson proposed the fluid mosaic model of membrane structure, which is now generally accepted as the basic paradigm for the organization of all biological membranes. In this model, membranes are viewed as two-dimensional fluids in which proteins are inserted into lipid bilayers.
Singer and Nicolson distinguished two classes of membrane-associated proteins, which they called peripheral and integral membrane proteins. Peripheral membrane proteins were operationally defined as proteins that dissociate from the membrane following treatments with polar reagents, such as solutions of extreme pH or high salt concentration, that do not disrupt the phospholipid bilayer. Once dissociated from the membrane, peripheral membrane proteins are soluble in aqueous buffers. These proteins are not inserted into the hydrophobic interior of the lipid bilayer. Instead, they are indirectly associated with membranes through protein-protein interactions. These interactions frequently involve ionic bonds, which are disrupted by extreme pH or high salt.
In contrast to the peripheral membrane proteins, integral membrane proteins can be released only by treatments that disrupt the phospholipid bilayer. Portions of these integral membrane proteins are inserted into the lipid bilayer, so they can be dissociated only by reagents that disrupt hydrophobic interactions. The most commonly used reagents for solubilization of integral membrane proteins are detergents, which are small amphipathic molecules containing both hydrophobic and hydrophilic groups. The hydrophobic portions of detergents displace the membrane lipids and bind to the hydrophobic portions of integral membrane proteins. Because the other end of the detergent molecule is hydrophilic, the detergent-protein complexes are soluble in aqueous solutions.
Many integral proteins are transmembrane proteins, which span the lipid bilayer with portions exposed on both sides of the membrane. These proteins can be visualized in electron micrographs of plasma membranes prepared by the freeze-fracture technique. In these specimens, the membrane is split and separates into its two leaflets. Transmembrane proteins are then apparent as particles on the internal faces of the membrane.
The membrane-spanning portions of transmembrane proteins are usually α helices of 20 to 25 hydrophobic amino acids that are inserted into the membrane of the endoplasmic reticulum during synthesis of the polypeptide chain. These proteins are then transported in membrane vesicles from the endoplasmic reticulum to the Golgi apparatus, and from there to the plasma membrane. Carbohydrate groups are added to the polypeptide chains in both the endoplasmic reticulum and Golgi apparatus, so most transmembrane proteins of the plasma membrane are glycoproteins with their oligosaccharides exposed on the surface of the cell.
Studies of red blood cells have provided good examples of both peripheral and integral proteins associated with the plasma membrane. The membranes of human erythrocytes contain about a dozen major proteins, which were originally identified by gel electrophoresis of membrane preparations. Most of these are peripheral membrane proteins that have been identified as components of the cortical cytoskeleton, which underlies the plasma membrane and determines cell shape. For example, the most abundant peripheral membrane protein of red blood cells is spectrin, which is the major cytoskeletal protein of erythrocytes. Other peripheral membrane proteins of red blood cells include actin, ankyrin, and band 4.1. Ankyrin serves as the principal link between the plasma membrane and the cytoskeleton by binding to both spectrin and the integral membrane protein band 3. An additional link between the membrane and the cytoskeleton is provided by band 4.1, which binds to the junctions of spectrin and actin, as well as to glycophorin (the other major integral membrane protein of erythrocytes).
The two major integral membrane proteins of red blood cells, glycophorin and band 3, provide well-studied examples of transmembrane protein structure. Glycophorin is a small glycoprotein of 131 amino acids, with a molecular weight of about 30,000, half of which is protein and half carbohydrate. Glycophorin crosses the membrane with a single membrane-spanning α helix of 23 amino acids, with its glycosylated amino-terminal portion exposed on the cell surface. Although glycophorin was one of the first transmembrane proteins to be characterized, its precise function remains unknown. In contrast, the function of the other major transmembrane protein of red blood cells is well understood. This protein, originally known as band 3, is the anion transporter responsible for the passage of bicarbonate (HCO3-) and chloride (Cl-) ions across the red blood cell membrane. The band 3 polypeptide chain is 929 amino acids and is thought to have 14 membrane-spanning α-helical regions. Within the membrane, dimers of band 3 form globular structures containing internal channels through which ions are able to travel across the lipid bilayer.
Because of their amphipathic character, transmembrane proteins have proved difficult to crystallize, as required for three-dimensional structural analysis by X-ray diffraction. The first transmembrane protein to be analyzed by X-ray crystallography was the photosynthetic reaction center of the bacterium Rhodopseudomonas viridis, whose structure was reported in 1985 (Figure 12.7). The reaction center contains three transmembrane proteins, designated L, M, and H (light, medium, and heavy) according to their apparent sizes indicated by gel electrophoresis. The L and M subunits each have five membrane-spanning α helices. The H subunit has only a single transmembrane α helix, with the bulk of the polypeptide chain on the cytosolic side of the membrane. The fourth subunit of the reaction center is a cytochrome, which is a peripheral membrane protein bound to the complex by protein-protein interactions.
Although most transmembrane proteins span the membrane by α-helical regions, this is not always the case. A well-characterized exception is provided by the porins—a class of proteins that form channels in the outer membranes of some bacteria. Many bacteria, including E. coli, have a dual membrane system in which the plasma membrane (or inner membrane) is surrounded by the cell wall and a distinct outer membrane (Figure 12.8). In contrast to the plasma membrane, the outer membrane is highly permeable to ions and small polar molecules (in the case of E. coli, with molecular weights up to 600). This permeability results from the porins, which form open aqueous channels through the lipid bilayer. As discussed in Chapter 10, proteins related to the bacterial porins are also found in the outer membranes of mitochondria and chloroplasts.
Structural analysis has indicated that the porins do not contain hydrophobic α-helical regions. Instead, they cross the membrane as β barrels, in which 16 β sheets fold up into a barrel-like structure enclosing an aqueous pore. The side chains of polar amino acids line the pore, whereas side chains of hydrophobic amino acids interact with the interior of the membrane. The porin monomers associate to form stable trimers, each of which contains three open channels through which polar molecules can diffuse across the membrane.
In contrast to transmembrane proteins, a variety of proteins (many of which behave as integral membrane proteins) are anchored in the plasma membrane by covalently attached lipids or glycolipids (Figure 12.10). Members of one class of these proteins are inserted into the outer leaflet of the plasma membrane by glycosylphosphatidylinositol (GPI) anchors. GPI anchors are added to certain proteins that have been transferred into the endoplasmic reticulum and are anchored in the membrane by a C-terminal transmembrane region (see Figure 9.16). The transmembrane region is cleaved as the GPI anchor is added, so these proteins remain attached to the membrane only by the glycolipid. Since the polypeptide chains of GPI-anchored proteins are transferred into the endoplasmic reticulum, they are glycosylated and exposed on the surface of the cell following transport to the plasma membrane.
Other proteins are anchored in the inner leaflet of the plasma membrane by covalently attached lipids. Rather than being processed through the secretory pathway, these proteins are synthesized on free cytosolic ribosomes and then modified by the addition of lipids. These modifications include the addition of myristic acid (a 14-carbon fatty acid) to the amino terminus of the polypeptide chain, the addition of palmitic acid (16 carbons) to the side chains of cysteine residues, and the addition of prenyl groups (15 or 20 carbons) to the side chains of carboxy-terminal cysteine residues In some cases, these proteins (many of which behave as peripheral membrane proteins) are targeted to the plasma membrane by positively charged regions of the polypeptide chain as well as by the attached lipids. These positively charged protein domains may interact with the negatively charged head groups of phosphatidylserine on the cytosolic face of the plasma membrane. It is noteworthy that many of the proteins anchored in the inner leaflet of the plasma membrane (including the Src and Ras proteins illustrated in Figure 12.10) play important roles in the transmission of signals from cell surface receptors to intracellular targets, as discussed in the next chapter.

