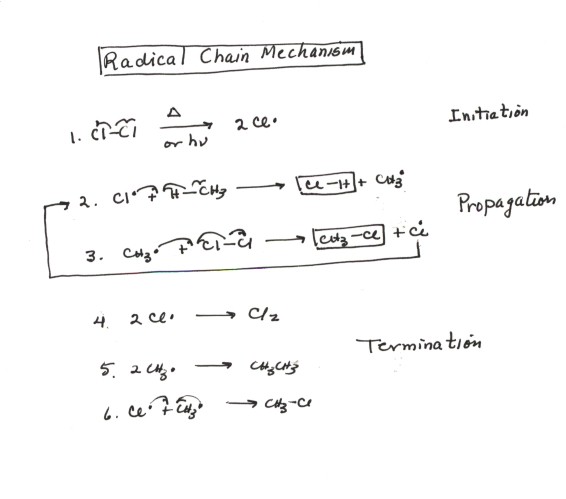
The steps of free radical reactions in 2022
This is one of the best depictions of the steps of free radical reactions I have seen. It shows what can go on in this reactions and how we get from starting material to desired final product.
Radical reactions: a quick overview first. A radical reaction is a reaction which occurs by a free radical mechanism (duh) and results in the substitution of one or more of the atoms or groups present in the substrate by different atoms or groups. Homolysis is the process by which one makes two new radicals by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond. Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light.
Initiation = One neutral provides two radicals.
This is what starts the entire reaction. This is also the only initiation step that can occur, as CH4 is not going to break off an H*. Remember that we need light or heat or some sort of radical initiator to start the initiation step in a radical reaction.
Propagation = 1 neutral + 1 radical provides a different neutral and a different radical.
In this reaction, the most likely propagation is chlorine abstracting a proton from methane to give HCl and the methyl radical. The next step is where the methyl radical breaks up two Cl atoms. What I really like about this depiction is that it shows that the Cl* from reaction 3 can be recycled back into step 2. This means that the reaction is self-propagating. This also means that IN THEORY you could have one initiation reaction, followed by a bunch of different propagations, ending with one termination reaction. Of course, in real life, for many reasons, this does not happen as there are lots of differnt initiation reactions.
Termination = 2 radicals providing one neutral.
The part to remember here is that any two radicals can get together to terminate the reaction and form a neutral species. Since we have 2 types of radicals in the reaction (Cl* and CH3*) , there are three combinations of potential termination steps. Reaction 4 gives us back starting material, so it is fine. Reaction 6 gives us product, so it is also fine. Reaction 5 give us a byproduct, which strangely enough can replace methane in the propagation step and give us another by-product.
Think about this picture and figure out all of the side reactions that might occur to fowl up the reaction. Then, (for you advanced students) think about what ways exist that you can minimize those side reactions.
Here is the quick summary of radical reactions:
- Initiation = 1 neutral provides two radicals.
- Propagation = 1 neutral + 1 radical provides a different neutral and a different radical.
- Termination = 2 radicals providing one neutral.
Hope this was helpful to you all, and as always, happy reacting.