What are strong nucleophiles?
Strong nucleophiles:
Strong nucleophiles…this is why molecules react. The nucleophilic site of the nucleophile is the region of a molecule that is reactive and has the electron density.
Strong nucleophiles are VERY important throughout organic chemistry, but will be especially important when trying to determine the products of elimination and substitution (SN1 vs SN2) reactions. In fact, there is not a more important part of an organic chemistry reaction than the nucleophile and the electrophile. So, let’s look at what makes strong nucleophiles:
There are generally three factors to remember when discussing how nucleophilic a reactant is:
1) Size – Generally (but not always) the more linear and/or smaller the nucleophile, the more nucleophilic it will be. This is because it can react at more sites and will not be sterically hindered if it is smaller or linear. Remember, smaller nucleophiles can fit into more places, therefore will be able to react at more places and will necessarily be more nucleophilic. This has a lot to do with sterics. You will hear a lot about bulky bases, which are nucleophilic but too darn big to be a nucleophile and can only be a base.
2) Electronegativity– The more electronegative an atom is, the less nucleophilic it will be. This is because more electronegative atoms will hold electron density closer, and therefore will be less likely to let that electron density participate in a reaction. We see this in calculations and experiments that show nucleophilicity decreases as you get closer to fluorine on the periodic table (C > N > O > F).

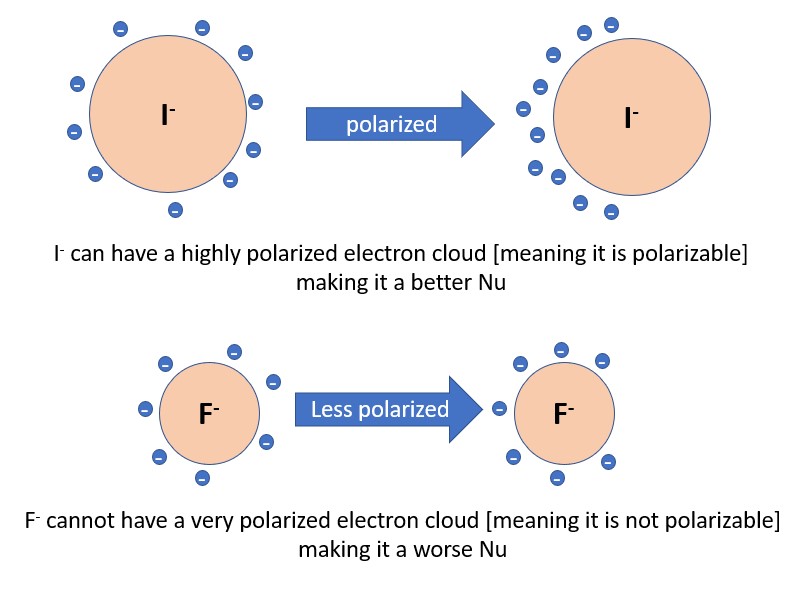
3) Polarizability– The more polarizable an atom is, the more nucleophilic it will be. Polarizability is defined as the ability to distort the electron cloud of an atom, which allows it interact with a reaction site more easily. Generally, polarizability increases as you travel down a column of the periodic table (I > Br > Cl > F)
Below is a table of relative nucleophilic strength. This is relative because nucleophilic strength is also dependent on other factors in the reaction, such as solvent. I am not a huge fam of memorizing charts, but this might be a good one to know pretty well.
| VERY Good nucleophiles | HS–, I–, RS– |
| Good nucleophiles | Br–, HO–, RO–, CN–, N3– |
| Fair nucleophiles | NH3, Cl–, F–, RCO2– |
| Weak nucleophiles | H2O, ROH |
| VERY weak nucleophiles | RCO2H |
As shown above, as a general rule, the anion of a reactant will be a better nucleophile than the neutral form. (i.e. RCO2– is a better nucleophile than RCO2H)
But nucleophiles are also bases?
Think about it for a second….good nucleophiles (as shown above) can have a negative charge and will almost always have a lone pair. Bases accept protons, with a negative charge or lone pair. [gasp] So it makes sense there will be at least some overlap between bases and nucleophiles. This is a major consideration when looking at SN vs E reactions.
Here are a couple of good rules to remember:
- Bases will not be good nucleophiles if they are really bulky or hindered. A variety of amine bases can be bulky and non-nucleophilic.
2. Nucleophiles will not be good bases if they are highly polarizable. I- is the best example of this. Great nucleophile, really poor base.
Why do we care about strong nucleophiles?
Organic chemistry is all about reactions. We really need to know what is nucleophilic and what is not so that we can determine what is going to react at the electrophilic site. If you know this, you can predict the products of organic chemistry reactions, even ones that you have not seen before.
The next step is to learn about electrophiles.
Please visit our recent post on this topic –> Electrophilic addition. Not to humble brag, but it is pretty good.
For more information on this and other topics of organic chemistry interest, please visit organic chemistry
Reference: Nucleophilic strength


thank you so much for the informations
they’ve been so useful
The poor nucleophiles is more favor to Sn1 reaction than Sn2 reaction. Is my statement correct?
Yes!
Sn1 proceed faster in more polar solvent compare to Sn2.
if i not mistaken.
how does base strength correlate with nucleophile strength?
all ready use to website more
Thank you so much for this!!!!!
So, would R-O-NH2 be a fair nucleophile or a weak nucleophile? I’m thinking it would be weaker than NH3 because of the oxygen, but I’m not sure.
this is about to help me on my orgo exam yesss
If the iodide ion is a stronger nucleophile than the hydroxide ion, why does the latter displace the former in a reaction involving aqueous Sodium hydroxide and alkyl iodide?
Aqueous NaOH protonates OH group to make it a good leaving group, H2O. Than iodide is able to replace OH group.
true
Pingback: Electrophiles and Electrophilic Reactions: What makes a good electrophile? « Organic Chemistry made easy
Hi,
I am quite confused I ampretty sure in an SN2reaction I- would be a good electrophile not nucelophile?
size and polarizable effects are contracdictory,if size of the atom is larger more polarizablity is increases, therefore larger the size nucleophilicity increases. for (CH3)3C- > (CH3)2N->CH3O-
and also C->N->O->F- C size is larger than N,O and F.
I->Br->Cl->F- I- is larger in size than Br-, Cl- and F-