What is a hydrogen bond?
A hydrogen bond is an electrostatic attraction between an electronegative atom (one that has lone pair electrons) and a hydrogen atom bound to an electronegative atom.

Because the electronegative atom has lone pair electrons and steals some electron density from other places, it takes on a partial negative charge, symbolized by d-. Conversely, the hydrogen atom bound to that electronegative atom has some of its electrons drawn away, making it partially positive, or d+. When two of these molecules interact in solution, the d+ from hydrogen is attracted to the d- of the electronegative atom and a hydrogen bond is formed.
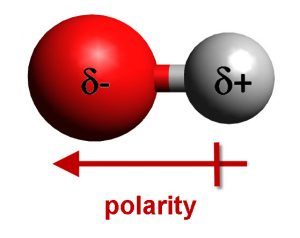
In contrast to a covalent bond, where two atoms formally share the two electrons in the bond, the two electrons of the hydrogen bond completely reside on the electronegative atom and are only attracted to the hydrogen atom. This makes a hydrogen bond much weaker than a covalent bond. Symbolically, we show a hydrogen bond as a dashed or dotted line, instead of a full line, to remind the user that it is not a formal bond.
So, how strong is a hydrogen bond?
The answer is not very. Remember, this is not a “bond” in the traditional sense, it is an electrostatic attraction. The hydrogen bond has only 5% or so of the strength of a covalent bond. However, when many hydrogen bonds can form between two molecules (or parts of the same molecule), the resulting union can be sufficiently strong as to be quite stable. The length of the hydrogen bond varies slightly, depending on what the electronegative atom is. In water, the length is generally accepted to be 1.98A.
One of the best examples of the hydrogen bonding is water. Oxygen is the electronegative atom here and provides the electrons for the hydrogen bond from one of its two lone pairs. One of the two hydrogen atoms on water will obviously be the electron acceptor. This means that each water molecule can have up to four hydrogen bonds at any one time, with two bonds to each lone pair and two bonds to each hydrogen atom.
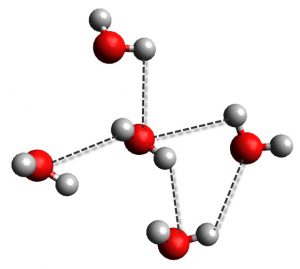
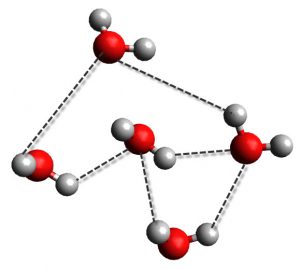
Moreover, hydrogen bonding is quite dynamic, meaning that hydrogen bonds are constantly changing. In water, that means that they are constantly breaking and forming between the different water molecules. The pictures above are just snapshots in time, because the process of forming and breaking bonds happens way to fast to see or measure.
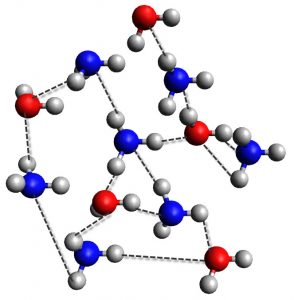
But it occurs in other places too. Now that we know hydrogen bonding occurs in water, it is not that great a stretch to think that it would also occur in alcohols. Moreover, hydrogen bonding occurs in amines, carbonyls, and other organic chemistry and biological molecules. Below is a representation of the hydrogen bonding that might occur between water and ammonia. Notice that hydrogen can bond with nitrogen (once) or oxygen (twice).
Why do we care about such a weak little bond?
- Hydrogen bonding affects the physical properties of molecules, such as BP. Hydrogen bonding tends to increase boiling point, when compared to molecules of a similar size that don’t hydrogen bond.
- Hydrogen bonding makes liquids into better solvents. This is because it can adhere to solutes better through this bonding….who would have thought?
- Hydrogen bonding affects conformations, such as in enols. This one is pretty cool. Generally, if a molecule can be in either conformation, it will choose the keto form because it is more stable. However, if there is a highly-stabilizing hydrogen bond, it might be more stable in the enol form.
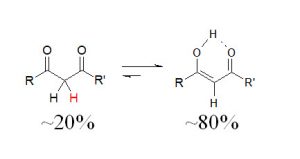
- Hydrogen bonding greatly affects our biology. Numerous examples in biochemistry show how important hydrogen bonding is. In DNA, hydrogen bonds between nitrogenous bases in nucleotides on the two strands of (guanine pairs with cytosine, adenine with thymine) give rise to the double-helix structure that is crucial to the transmission of genetic information. In proteins, protein folding is reliant on hydrogen bonding. Receptor-substrate binding cannot generally occur without hydrogen bonding. Further, as stated above, it keeps water as a liquid at much lower temperatures than one would predict based on the size/weight of the molecule. We clearly couldn’t live without hydrogen bonds.

Reference: Hydrogen bonds

